Diffusion and 3 Experiments
Diffusion
The tendency of solid, liquid, or gas to spread spontaneously and uniformly in any medium is called diffusion. In the diffusion process, a solid, liquid, or gas moves spontaneously from a place of higher concentration to a lower concentration.
Remember, what happens if you leave a perfume bottle with its lid open in the corner of a room? Within some time, you will find the whole room smelling of perfume. This is an instance of diffusion. If a matter takes less time to spread, its diffusion rate is higher, and vice versa.
Now, read through the following experiments.
Experiment - 1
At room temperature, take some pure water in a glass jar. Add a small quantity of solid pink Potassium permanganate (KMnO4) into it. You will see, after some time, that the KMnO4 grains are dissolving into a pink solution. Indeed, the KMnO4 particles are getting motion and scattering freely in the water. In this case, some solid matter is diffused in water or liquid. The rate of diffusion of solid matter in water is slow, however, if heat is applied, the rate will increase.
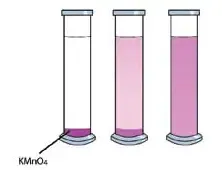
Similarly, if we do the experiment of diffusion of Potassium permanganate in hot water, the water will turn into a pink solution faster. In this case, the KMnO4 particles will get added motion due to heat and scatter faster. This shows that the presence of heat increases the rate of diffusion of solids.
Experiment - 2
At room temperature, put some pure water in a beaker and add some liquid blue to it. Within a few minutes, you will see that the water has turned blue. That means, the particles of blue solution or some liquid have diffusion in the water.
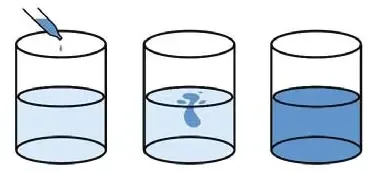
The temperature being the same, it has taken the liquid less time to diffuse in another liquid than solid KMnO4.
That means the rate of diffusion of liquid matter in another liquid is faster than in solid matter. The presence of heat will increase the rate. At room temperature, or in the presence of heat, gaseous matter diffuses the fastest.
Experiment - 3: Diffusion of two gases
Take a glass tube with both faces open. Take two pieces of cotton. Soak a piece of cotton in concentrated hydrochloric acid (HCl solution and soak the other in ammonium hydroxide (NH4OH) solution. Now close the glass tube fixing each of the cottons to a side of it. Here hydrogen chloride gas from the HCl solution and ammonia gas from NH4OH the solution will get diffused. Within a few moments, you will see a white smoke filling up the tube. It is ammonium chloride (NH4Cl), produced in the reaction between hydrogen chloride gas and ammonia gas. The white smoke will not be positioned in the center of the tube; it will be nearer to the hydrochloric acid solution. That means at the same given time, hydrogen chloride gas has gone a lesser distance than ammonia. That also proves that ammonia gas spreads faster than hydrogen chloride gas because of its faster rate of diffusion. **
The reason behind this is the difference in their atomic masses. A gas with a lesser atomic mass will have a better diffusion rate. Here ammonia gas (atomic mass 17) has spread faster than hydrogen chloride gas (atomic mass 36.5).

The atomic mass of H2, He, N2, O2, and CO2 gases are 2, 4, 28, 32, and 44 respectively. since hydrogen is the gas with the least atomic mass, its rate of diffusion is faster than others while carbon dioxide being the most, is the slowest.
Chemistry
Secondary Chemistry
- Chapter - 1 : Concept of Chemistry
- Chapter - 2 : States of Matter
- Chapter - 3 : Structure of Matter
- Chapter - 4 : Periodic Table
- Chapter - 5 : Chemical Bond
- Chapter - 6 : Concept of Mole and Chemical Counting
- Chapter - 7 : Chemical Reactions
- Chapter - 8 : Chemistry and Energy
- Chapter - 9 : Acid - Base Balance
- Chapter - 10 : Mineral Resources: Metal - Nonmetal
- Chapter - 11 : Mineral Resources: Fossils
- Chapter - 12 : Chemistry in Our Lives



