Atom Definition, What is atom? How big is an atom?
Atom Definition
An atom is the basic building block of matter. It is the smallest unit into which matter can be divided without losing its identity as a chemical element.
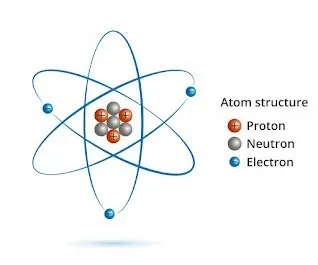
What is atom?
An atom is the fundamental building block of everything around you, from the air you breathe to the chair you're sitting on. It's the smallest unit of matter that still retains the properties of a specific chemical element.
Atom Examples
- Hydrogen (H): The simplest atom, with just one proton and one electron. It's the most abundant element in the universe.
- Helium (He): Has two protons, two neutrons, and two electrons. Helium is the second-lightest element and is often used in balloons due to its low density.
- Lithium (Li): Contains 3 protons, 4 neutrons, and 3 electrons. Lithium is an alkali metal that is soft and reactive.
- Carbon (C): An essential element for life on Earth. It has 6 protons, 6 neutrons (in the most common isotope), and 6 electrons. Carbon atoms can bond with each other in various ways to form a vast number of molecules.
- Oxygen (O): Essential for respiration and makes up about 21% of the Earth's atmosphere. An oxygen atom has 8 protons, 8 neutrons (in the most common isotope), and 8 electrons.
- Iron (Fe): An abundant element in the Earth's crust and core. It has 26 protons, 30 neutrons (in the most common isotope), and 26 electrons. Iron is important for many biological processes.
- Gold (Au): A precious metal known for its luster and ductility. It has 79 protons, 118 neutrons (in the most common isotope), and 79 electrons.
What does an atom look like?
We cannot directly see an atom because they are incredibly tiny. The diameter of a hydrogen atom, the simplest atom, is about 100 picometers (pm). To put that in perspective, a human hair is roughly 80,000 pm in diameter!
Early models of the atom depicted electrons traveling in neat, circular paths around the nucleus, like planets around the sun. This is known as the Bohr model.
However, this model is not entirely accurate. Scientists now understand that electrons exist in a cloud of probability around the nucleus, rather than a definite path. The area where an electron is most likely to be found is called an electron orbital.
How big is an atom?
Atoms are incredibly small, way too small to be seen with the naked eye or even a regular microscope. They exist in the realm of picometers (pm).
Here's how tiny an atom is:
- Size in picometers: An atom is typically around 100 picometers (pm) across.
- Comparison to human hair: Human hair is about 80,000 picometers wide. So, if you could line up 800 million atoms side-by-side, they would only equal the width of a single human hair!
- Analogy: Imagine a marble representing the Earth. On that scale, an atom would be about the size of a grape!
It's important to note that the size of an atom can vary slightly depending on the element. But, 100 picometers is a good general estimate.
What is the mass number of an atom?
Atoms are incredibly tiny, existing in a world measured in picometers (pm), which are trillionths of a meter (1 pm = 0.000 000 000 000 001 m). Here's how to grasp their small size:
- Size in picometers: An atom typically ranges around 100 picometers in diameter.
- Comparison to a human hair: A human hair is roughly 80,000 picometers wide. Imagine lining up 800 million atoms side-by-side, and they'd only equal the width of a single hair!
- Analogy: If a marble represented Earth, an atom would be about the size of a grape!
What happens if you split an atom?
Splitting an atom, also known as nuclear fission, is a process that releases a tremendous amount of energy. Here's what happens:
- The Process: A neutron collides with the nucleus of a heavy atom, like uranium-235.
- The Split: The unstable nucleus absorbs the neutron and splits into two smaller nuclei, called fission products.
- Energy Release: During the split, a significant amount of energy is released in several forms:
- Kinetic Energy: The fission products fly apart at high speeds due to the repulsive force between them. This kinetic energy can be converted into heat.
- Electromagnetic Radiation: Gamma rays, a form of high-energy light, are also emitted during fission.
- Neutrons: Several neutrons are released in addition to the initial one that caused the split. These neutrons can trigger further fissions in other atoms, creating a chain reaction.
Chain Reaction: This chain reaction is crucial in nuclear power plants. The released neutrons can cause fission in other uranium atoms, releasing even more neutrons and continuing the process. This controlled chain reaction generates a large amount of heat, which is then used to create steam and drive turbines for electricity generation.
Where is most of the mass of an atom located?
Most of the mass of an atom is located in its nucleus. The nucleus is incredibly dense and despite being a tiny part of the atom, it holds the vast majority of the atom's weight.
Here's a breakdown of why the nucleus is so much more massive:
- Nucleus: Composed of protons and neutrons. Protons have a positive charge and are much heavier than electrons (around 1,836 times the mass of an electron). Neutrons are also relatively heavy and have a mass similar to protons.
- Electrons: Orbit the nucleus in shells and are much, much lighter than protons or neutrons. The mass of an electron is negligible compared to the mass of a proton or neutron.
How small is an atom?
Atoms are incredibly tiny, existing in a realm beyond what we can perceive with our unaided senses.
Here's how to grasp their minuscule size:
- Size in picometers: An atom typically measures around 100 picometers (pm) in diameter. To truly understand how small a picometer is, consider this: one meter equals 1 trillion picometers!
- Comparison to a human hair: A human hair, by comparison, is roughly 80,000 picometers wide. Imagine lining up 800 million atoms side-by-side, and they'd only span the width of a single hair!
What is the difference between an atom and a molecule?
The main difference between an atom and a molecule lies in their composition and how they exist:
Atom:
- The fundamental building block of matter.
- The smallest unit that retains the properties of a specific chemical element.
- Exists independently as a single unit.
- Composed of a central nucleus containing protons (positively charged) and neutrons (no charge), surrounded by electrons (negatively charged) in orbitals.
- The number of protons determines the element (e.g., all atoms with 6 protons are carbon atoms).
Molecule:
- A collection of two or more atoms held together by chemical bonds.
- The smallest unit represents a compound (a substance made from two or more different elements chemically bonded).
- Doesn't necessarily exist independently. Can exist as single molecules or bonded together in structures.
- The composition and arrangement of atoms within a molecule determine its properties, which are often different from the individual atoms themselves.
The atom is the smallest unit of matter that retains the properties of a chemical element. It is the basic building block of chemistry and cannot be divided further without releasing charged particles.



